myeloMATCH Overview
|
MyeloMATCH is a group of clinical trials for people with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS). These trials test new precision medicine treatments for patients with myeloid cancers, from diagnosis through multiple stages of their treatment journey. |
myeloMATCH Links Additional Resources |
Known as an “umbrella trial,” myeloMATCH tests multiple treatments in people who have the same type of cancer (AML or MDS) but different genomic changes (or biomarkers) in their cancer cells.
Rapid Testing
For those interested in joining myeloMATCH, the first step is to enroll in the MYELOMATCH screening protocol (also known as the myeloMATCH Master Screening and Reassessment Protocol). Using the most advanced testing, this protocol guides the evaluation of newly diagnosed patients and assigns them to a treatment trial or tracks their standard-of-care treatment.
All patients will have blood and bone marrow specimens collected and tested, at no cost. The testing looks for certain biomarkers (clinical, cytogenetic, or molecular features) that may be causing the cancer to grow and which may serve as specific targets for treatment.
Results of a patient’s initial biomarker test will be returned to their doctor within around three days. These results will be used to assign the patient to a myeloMATCH treatment trial, if a trial is available that matches their biomarkers. If there is not a matching treatment trial, patients will go on to receive standard-of-care treatment with their physician and will continue to be followed on myeloMATCH via the Tier Advancement Pathway.
Treatment Trials
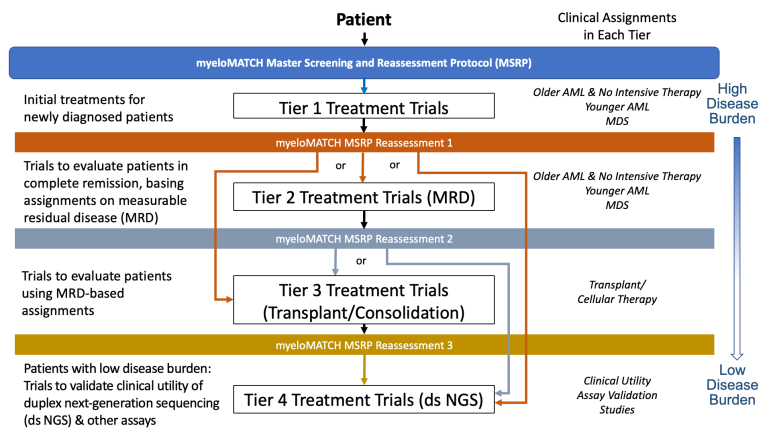
MyeloMATCH trials are organized into four “tiers” according to the stage of the patient’s cancer treatment journey they address.
- Tier 1 trials offer initial induction therapy for newly diagnosed patients
- Tier 2 trials treat patients for residual disease after initial therapy
- Tier 3 trials offer consolidation therapy or transplant
- Tier 4 trials treat progressively lower levels of residual disease burden that may remain
As an enrolled patient completes a course of treatment, new specimens will be collected and sent for testing. If that testing shows the patient's disease has been significantly reduced, a patient may be assigned to a new trial in a later tier. The myeloMATCH tier structure is illustrated below.
Patients also have a standard-of-care option if their treatment site does not have a trial open to match the patient’s genomic markers. Enrolling on the myeloMATCH standard-of-care option means that when a patient completes that treatment, they may be able to join a genomically matched myeloMATCH trial at a later tier of treatment.
As a patient proceeds through successive stages of treatment for AML or MDS, their disease burden is often progressively reduced. A long-term goal of myeloMATCH is to develop therapies that more precisely target this progressively reduced measurable residual disease (MRD). MyeloMATCH will use advanced biomarker tests to identify ultra low-level residual disease in patient specimens, to determine whether additional treatment may improve patient outcomes.
The NCI Precision Medicine Initiative
MyeloMATCH is one of three next-generation precision medicine trials the National Cancer Institute (NCI) has recently launched. The ComboMATCH trial was activated in 2023, and the ImmunoMATCH trial is now conducting a pilot study. As with these other precision medicine trials, all testing of patient samples for trial assignment will be done through the NCI’s Molecular and Immunologic Diagnostic Laboratory Network (MDNet).
MyeloMATCH is supported by the NCI, and the MYELOMATCH screening protocol is coordinated by the SWOG Cancer Research Network. MyeloMATCH includes treatment trials developed and conducted by Alliance for Clinical Trials in Oncology, Canadian Cancer Trials Group, ECOG-ACRIN Cancer Research Group, and SWOG Cancer Research Network. MyeloMATCH trials are conducted within the NCI’s National Clinical Trials Network and the NCI Community Oncology Research Program, and include collaborating personnel from BMT Clinical Trials Network, Children's Oncology Group, and NRG Oncology.